Carbon and its Compounds: Class 10 Science Notes
Carbon and its Compounds Class 10 Science Complete Notes. This note include all the excercise given in Carbon and its Compounds chapter of science book such as question answer, numericals, give reason, diagrammatic questions and define the following. This note is published for helping students to solve their problems.
If you find any mistakes or feel like giving any suggestions then feel free to comment down.
Related Searches
- What is carbon and its compounds class 10th?
- What is carbon and its compounds?
- What is carbon and its properties Class 10?How do you name a carbon compound class 10?
- Carbon and its Compounds Class 10 PDF
- Carbon and its compounds Class 10 Questions with Answers PDF
- Carbon and its Compounds Class 10
Chapter - 12
Carbon and its Compounds
📚EXERCISES
2. Differentiate Between:
a. Alkane and Alkene
Ans: The differences between alkane and alkene are as follows:
| Alkane | Alkene |
|---|---|
| In it carbon atoms are bounded with a single covalent bond | In it carbon atoms are bounded with a double covalent bond |
| It is represented by a formula : CₙH₂ₙ+2 | It is represented by a formula: CₙH₂ₙ |
| It is less reactive | It is more reactive |
b. Alkene and Alkyne
Ans: The differences between Alkene and Alkyne are as follows:
| Alkene | Alkyne |
|---|---|
| It has double covalent bond between carbon atoms |
It has triple covalent bond between carbon atoms |
| It is represented by a formula: CₙH₂ₙ |
It is represented by a formula: CₙH₂ₙ-2 |
| It is less reactive | It is more reactive |
c. Saturated and Unsaturated Hydrocarbon
Ans: The differences between Saturated and Unsaturated hydrocarbon are as follows:
| Saturated Hydrocarbon | Unsaturated Hydrocarbon |
|---|---|
| Carbon atoms are bonded together by a single covalent bond in them | Carbon atoms are bonded together by a triple or double covalent bond in them |
| They have only one group i.e alkane | They have two groups i.e alkene and alkyne |
| They are less active | They are more active |
d. Alkane and Alkyne
| Alkane | Alkyne |
|---|---|
| It has single covalent bond between carbon atoms | It has triple covalent bond between carbon atoms |
| It is represented by a formula : CₙH₂ₙ+2 | It is represented by a formula: CₙH₂ₙ-2 |
| It is less reactive | It is more reactive |
3. Give Reason:
a. Acetylene is an unsaturated hydrocarbon.
Ans: Acetylene is an unsaturated hydrocarbon because its two carbon atoms are bonded together in are a triple bond
b. Alkene are more reactive than alkane.
Ans: Alkene are more reactive than alkane because alkene contain carbon atoms which are bonded together by double covalent bond.
c. Methane gas is called marsh gas.
Ans: Methane gas is called Marsh because it is found at the surface of marshy place (a type of wetland).
d. Alkene are called olefins.
Ans: Alkene are called olefin because alkene reacts with chlorine and oily products are formed.
e. Propane is a saturated hydrocarbon.
Ans: Propane is a saturated hydrocarbon because Propane contains carbon atoms bonded with single covalent bond and it is chemically less reactive.
4. Answer the following questions:
a. Define saturated hydrocarbon? Write any three uses of methane.
Ans: A Saturated hydrocarbon are those hydrocarbons in which carbon atoms are bonded together by single covalent bond. The three uses of methane are as follows:
- It is used for making printing ink
- It is used in the form of LPG gas for domestic use
- It is used for making carbon black needed for paints and in rubber industries
b. Write the uses of alcohol.
Ans: The uses of alcohol are as follows:
- It is used as solvent to dissolve, oil, fat etc
- It is used as fuel
- It is used in spirit lamps
- It is used for dry cleaning
- It is used as anti-freeze for automobile radiation
c. Write the characteristics of homologous series.
Ans: The characteristics of homologous series are as follows:
- All members of this series show similar properties
- Each successive member of a homologous series differs by CH₂
- All members can be prepared by general method of preparation
- Molecular weight of all members of this series is in ascending order
d. Define functional group and alkyl group. What does IUPAC stand for ?
Ans: Functional group is defined as an atom or group of atoms (radical) which determines the chemical behaviour i.e physical and chemical properties of organic compounds.
The hydrocarbon unit derived by the removal of one hydrogen from alkane is called alkyl group.
IUPAC stands for International Union of Pure and Applied chemistry.
e. Write down the molecular and structural formula of Butane. State a Common use of butane.
Ans: Molecular formula of Butane : C₄H₁₀
Structural formula of Butane :
Common Use Of Butane
- It is used as raw material to manufacture synthetic rubber industries
f. "Acetylene is an unsaturated hydrocarbon" Justify the statement with it's molecular structure.
Ans: Acetylene is an unsaturated hydrocarbon because the carbon atom of acetylene are bonded together in a triple bond.
It's molecular structure is : H - C ≡ C - H
g. Give the structural formula of ethylene. What type of bond is found between carbon and hydrogen in this Compound?
Ans: The structure formula of ethylene is:
Double Covalent bond is found between carbon and hydrogen in this compound.
h. Define glucose? Write its two uses.
Ans: Glucose is one of the most common and abundant monosaccharides which is also called single sugar
The two uses of glucose are as follows:
- It is the primary source of energy for all body cells
- Liquid glucose is used as sweetener in bood
5. Diagrammatic Questions:
a. The structural formula of hydrocarbon is given. Answer the following questions.
i. Write the name and type of the given hydrocarbon.
Ans: The name of the given hydrocarbon is ethene (C₂H₄) and it is unsaturated by drocarbon.
ethene (C₂H₄) and it is unsaturated by drocarbon.
ii. Write down the name, structural formula and types of hydrocarbons formed in the reaction of the given hydrocarbon with hydrogen gas.
Ans: When the given hydrocarbon is reacted with hydrogen gas then it forms saturated hydrocarbon.
Name of hydrocarbon : Ethane
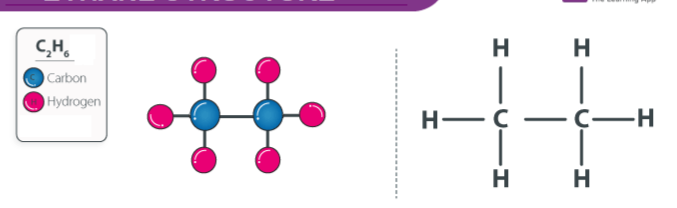 |
| Credit : Byju's |
b. The structural formula of a hydrocarbon is given. Answer the following questions.
i. Which compound is formed if all 'OH' groups are replaced by 'H' ?
Ans: If all 'OH' group are replaced by 'H' then Propane is formed.
ii. Give a use of compound formed in this way ?
Ans: It is used as a fuel in lighters.
c. A certain hydrocarbon has a structural formula as shown.
i. Is this a saturated hydrocarbon or unsaturated hydrocarbon ?
Ans: This is unsaturated hydrocarbon.
ii. If the above mentioned hydrocarbon is made to react with plenty of hydrogen, a new hydrocarbon will be formed. Name the hydrocarbon formed in this way and draw its structural formula:
Ans: The new formed hydrocarbon is propane.

d. What are the compounds of the following structural formula? Write the type of hydrocarbon on the basis of the bond. Write any one use of each.
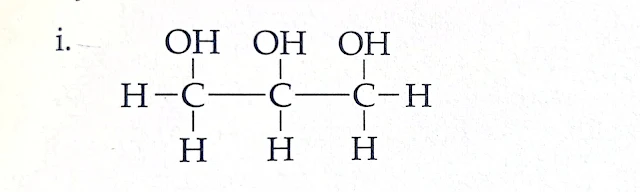
Name : Glycerol
Type : Saturated Hydrocarbon
Use : It is used as lubricants in watches

Name : Butene
Type : Unsaturated Hydrocarbon
Use : It is used as monomer for polybutene
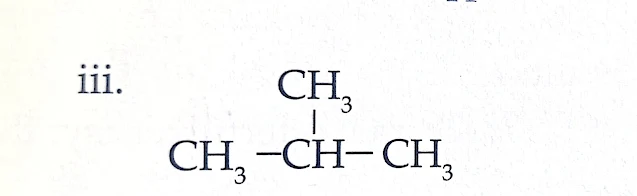
Name : Butane
Type : Saturated Hydrocarbon
Use : It is used as fuels for lighters
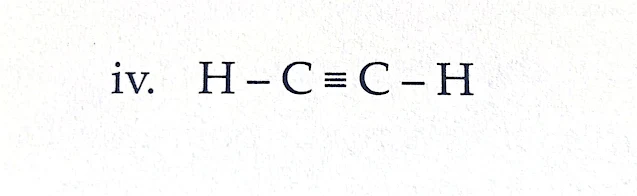
Type : Unsaturated Hydrocarbon
Use : It is used to prepare various organic compounds
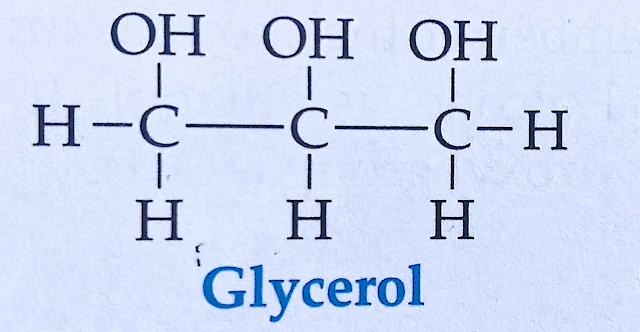
e. Sketch molecular structure of trihydric alcohol.


f. Define:
a. Organic Compound
Ans: Organic Compound are any chemical compound that Contains Carbon - hydrogen bond
b. Homologous Series
Ans: A series of organic compound which have the same functional group that can be represented by same general formula is called homologous series
c. Organic Chemistry
Ans: Branch of chemistry that deals with the study of hydrocarbon and their derivatives.
d. Hydrocarbon
Ans: Hydrocarbon is an organic compound of hydrogen and carbon
g.Write a note on:
a. Unsaturated hydrocarbon
Ans: The compounds in which carbon atoms are linked together by multiple bonds ( either double or triple bond ) are known as unsaturated hydrocarbons.
The nature of carbon-hydrogen bond is the same as for all saturated and unsaturated hydrocarbons. Ethylene, acetylene, propylene, allylene etc. are some examples of unsaturated hydrocarbons.
b. Alkenes
Ans: Alkenes are unsaturated hydrocarbons in which carbon atoms are bonded together by double covalent bonds (C = C). Alkenes contain two less hydrogen atoms compared to alkanes and are thus designated as unsaturated hydrocarbons. They are also called olefins because alkene reacts with chlorine and oily products are formed.
c. Alkynes
Ans: Alkynes are unsaturated hydrocarbons in which any two carbon atoms are link by a triple covalent bond in a molecule. Alkynes contain 4H- atoms less than that of the corresponding alkanes.
d. Glycerol
Ans: Glycerol is a kind of simplest trihydric alcohol. The word is derived from the word glyceros, meaning sweet. It is formed by the replacement of three hydrogen atoms of propane by three hydroxyl groups (-OH) The IUPAC name of glycerol is propane 1, 2, 3, triol.
e. Butane
Ans: Butane is the saturated hydrocarbon. It occurs in a natural gas and is found in petroleum mines. When butane is mixed with methane in high pressure, it forms a liquid fuel, which is called LPG or liquefied petroleum gas.
f. Alkyl Group
Ans: The hydrocarbon unit derived by the removal of one hydrogen atom from alkane is called alkyl group or alkyl radical. The name of an alkyl radical is written by replacing the ending -ane of the alkane with - yl.
If Class 10 Carbon & It's Compound Notes were helpful to you then feel free to share this notes with your friends & classmates.


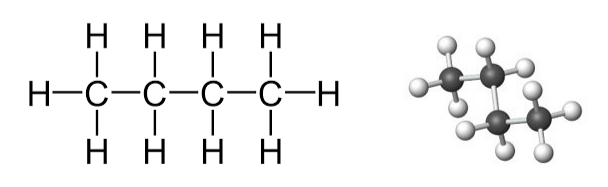







5 a ii. Answer
The hydrocarbon formed in the reaction of the given hydrocarbon with hydrogen gas is ethane.
It is a saturated type of hydrocarbon
Structural formula :
H H
H---C-------C----H
H H
Also give 17 18 19 notes
5 c ii.
The new formed hydrocarbon is propane.
Make structural formula of C3H8